Duly justified
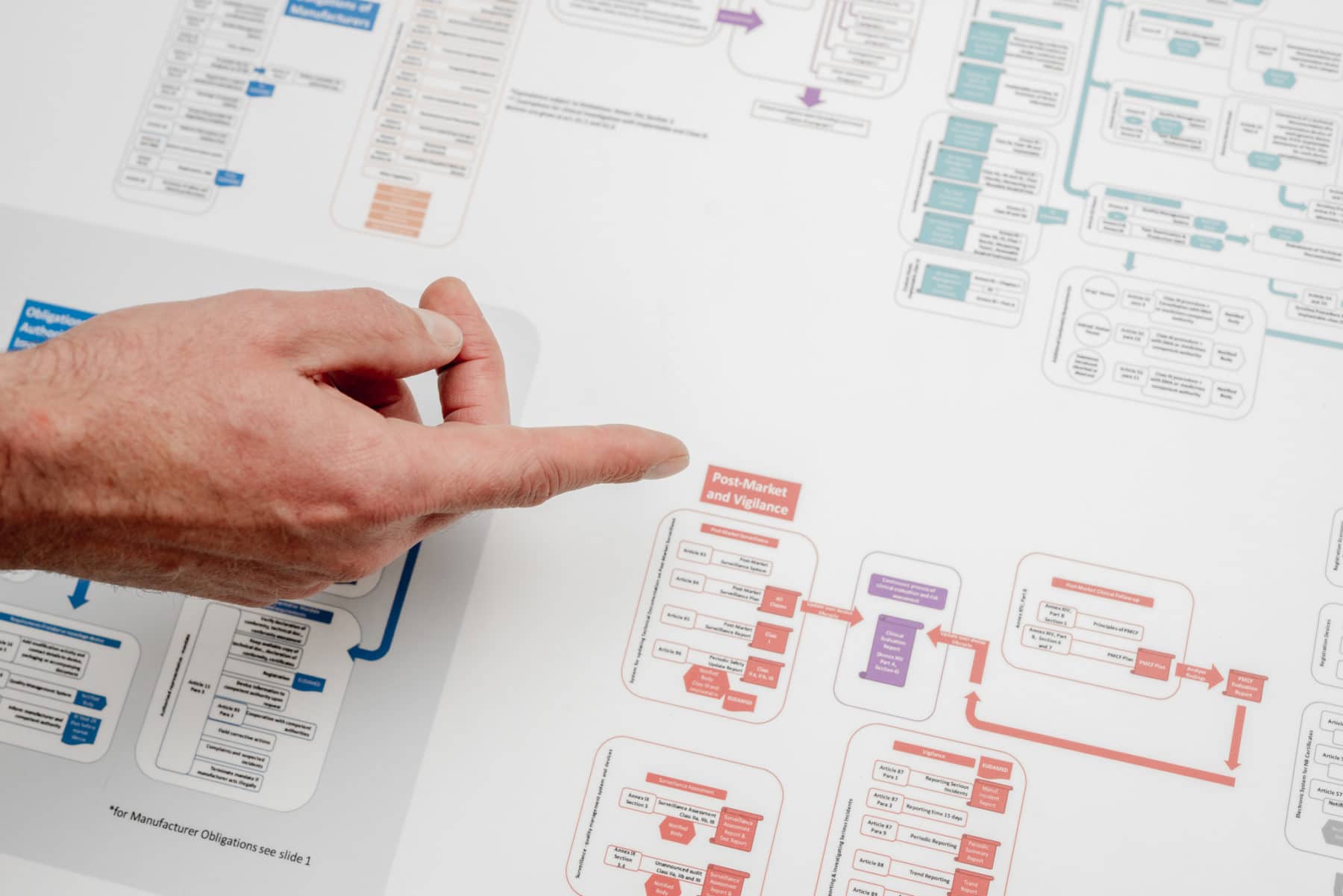
About two years ago, one of our customers asked us to help compiling the technical documentation for their MDD class I product. We set up the risk management and post-market surveillance procedures, documented and completed the risk management file, collected and provided evidence for all applicable essential MDD requirements and, of course, compiled all necessary technical data (including production procedures from our ISO 13485 certified production facility). Our customer took his responsibility: no shortcuts were taken here.
Whilst having their product on the market, our customer experienced some fuzziness about the classification of their device. Questions were asked by their customers and even by competitors about whether the device shouldn’t be a class IIa or IIb device. They also experienced difficulties formulating a straightforward answer to those questions. So they asked Unitron Regulatory a few months ago to help and write a statement about the classification of the device.
We took the device’s intended use and dove again into the MDD classification rules. We already knew that the device was actually a ‘borderline case’, so we had to dig into the exact wording used in the MDD, and their (sometimes not so clearly defined) meaning. What exactly is ‘diagnosis’? And what is meant by ‘monitoring’? Are we monitoring a patient if we only continuously measure certain body parameters, but not display any of those? By experience with lots of medical devices, we were convinced class I was correct, but we needed to sort out exactly how the rules would allow us to justify our view. And they did.
It is perfectly fine under European law to formulate your views to show compliance, as long as you can duly prove that safety is guaranteed within the limits as defined and communicated to users and authorities. So we wrote a rationale document for (our view on) the classification of our customer’s device. And right in time: just two weeks later the Dutch Inspection knocked on their door for a routine check. One of the items they asked for was the device’s classification and its rationale. To our delight, our rationale was accepted! Duly justified. Check.
You might be in a similar situation, needing help with classification or interpretation of other MDD or MDR parts. We would gladly help. Don’t go over these aspects too quickly. See our contact page to get in touch.